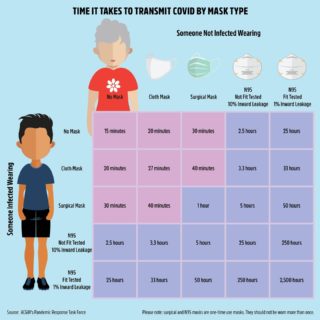
Before authorizing any vaccine, the FDA must ensure that it is both safe and effective. In order to guarantee public safety, rigorous tests are conducted before human trials, during human trials, and after the vaccine is approved.
There are 3 phases every vaccine must follow before FDA authorization.
- Phase 1: The vaccine is given to a small number of healthy adults and evaluated for safety, dosage, and side effects.
- Phase 2: The vaccine is given in various dosages to hundreds of adults from different backgrounds. This phase provides more information on short-term side effects and helps experts compare dosage amount against immune response.
- Phase 3: The vaccine is given to thousands of adults from a wide variety of ages, backgrounds, and health issues. Experts monitor effectiveness of the vaccine against the targeted disease as compared to those who have received a placebo (an inactive vaccine).
Even after authorization, the FDA continues to monitor the safety of any vaccine very closely and oversees all production of the vaccine.
SOURCE https://www.cdc.gov/vaccines/basics/test-approve.html (CDC)
Updated 12/16/2021